Background: Unanchored MAIC (Matching-Adjusted Indirect Comparison) is an ITC (Indirect Treatment Comparison) method adjusting for cross-trial heterogeneity in patient demographic or disease that are believed to be either prognostic or treatment effect modifiers. In this analysis, two trials for adults with secondary acute myeloid leukemia (AML) were compared by an unanchored MAIC.
Aims: The GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto)-SEIFEM (Sorveglianza Epidemiologica Infezioni nelle Emopatie) real-life study on the use of CPX-351 (Fianchi et al- Cancers 2023) was weighted for the aggregated patients characteristics from the standard arm(“7+3”) of the CPX-351 trial (cytarabine and daunorubicin Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia, Lancet et al - JCO 2018). This analysis aimed to test the feasibility to compare individual patients' data with aggregated published results and evaluate the rate of infections of CPX-351 in real life vs the “3+7” regimen and their impact on the survival outcomes.
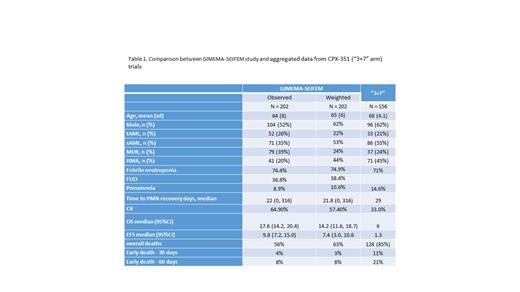
Methods: Patients-level data from GIMEMA-SEIFEM on the use of CPX-351 (n=202) and aggregated data from CPX-351 (“3+7” arm, n=156) trials were used to conduct an unanchored MAIC. GIMEMA-SEIFEM study included included all consecutive patients with AMLfrom 30 Italian hematologic centers who received at least 1 course of CPX-351 from July 2018 to June 2021 according to clinical practice. Patients from the GIMEMA-SEIFEM study were weighted to balance with baseline characteristics from the USA and Canada cohoort. Accordingly, weighted Overall and Event-free survival (w-OS, w-EFS) estimates, as well as rates of febrile neutropenia, pneumonia, CR, and the interval of PMN recovery, were computed.
Results: Four potential effect modifiers were identified and used for adjustment: age, sex, AML subtype (tAML, sAML, MRC), and prior HMA exposure. Median w-OS and w-EFS were 14.2 (95%CI: 11.6-18.7) and 7.4 (95%CI: 3.0-10.6) months, respectively. These estimates were slightly lower than those documented in the most recent report of the GIMEMA-SEIFEM trial (median OS 17.7 months and median EFS 9.8 months) and higher than the results obtained by the standard arm of the CPX-351 trial (median OS 5.9 months, median EFS 1.3 months).
Weighted rates of febrile neutropenia, pneumonia, CR, and interval of PMN recovery were comparable to the observed values and better than observed in the standard arm of the CPX-351 trial for all considered variables, except for febrile neutropenia (Table 1).
Conclusions: The MAIC method allowed a robust comparison of two clinical trials for the treatment of AML patients. After adjustment, survival outcomes of the real-life cohort were slightly lower than the observed estimates and higher than the observed in the standard arm of the CPX-351 trial. Pneumonia risk was confirmed lower in GIMEMA-SEIFEM CPX-351 matched group than in “3+7” arm. This pilot analysis underlined the potentiality of this statistical method. Indeed, it could be useful to compare with high accuracy studies with strong differences in the selection of patients.
Disclosures
Cattaneo:pfizer, jazz: Other: travel grants. Candoni:Pfizer: Consultancy; Astellas: Honoraria; Janssen: Honoraria; Incyte: Consultancy, Honoraria. Fracchiolla:Abbvie, Jazz, Pfizer, Amgen: Speakers Bureau; Abbvie, Jazz, Pfizer, Amgen: Other: travel grants. Zappasodi:Amgen, Pfizer, Abbvie, Astellas: Honoraria. Pagano:Pfizer: Honoraria; Novartis: Honoraria; Menarini: Honoraria; Moderna: Honoraria; AstraZeneca: Honoraria; Janseen: Honoraria; Jazz: Honoraria; Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal